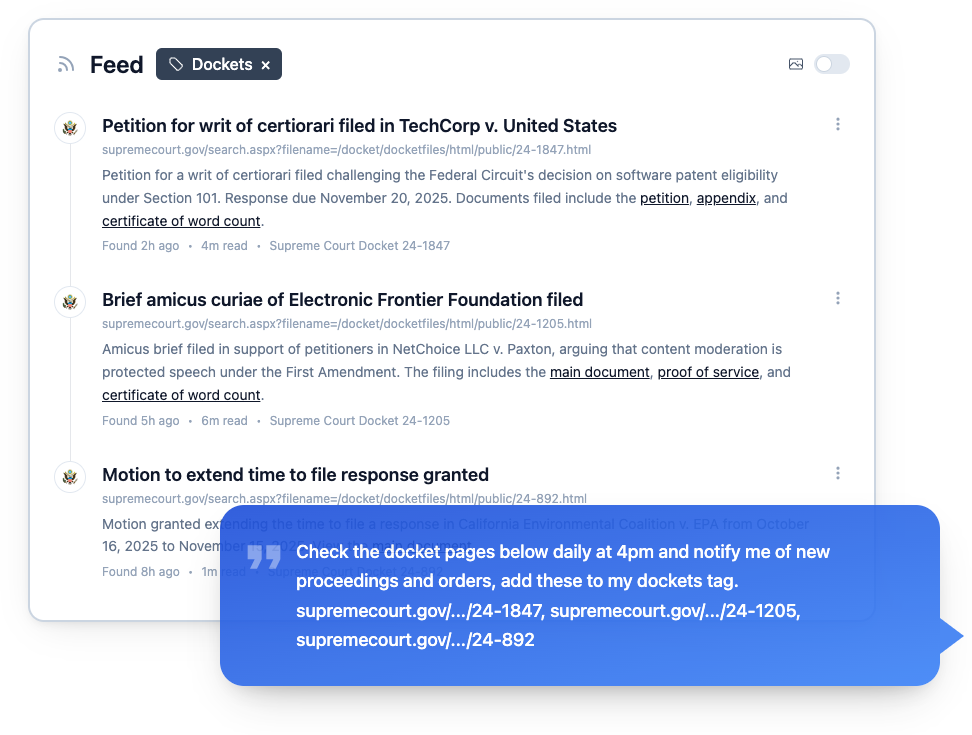
FDA COMPLIANCE MONITORING
Smart feeds for every Feeds for every FDA guidance document you care about you care about
Pharmaceutical and medical device companies can't afford to miss FDA updates. Changeflow monitors FDA.gov around the clock, alerting your compliance team to guidance changes, warning letters, and regulatory updates that affect your products.
Trusted by pharmaceutical and healthcare compliance teams
AI-powered FDA monitoring
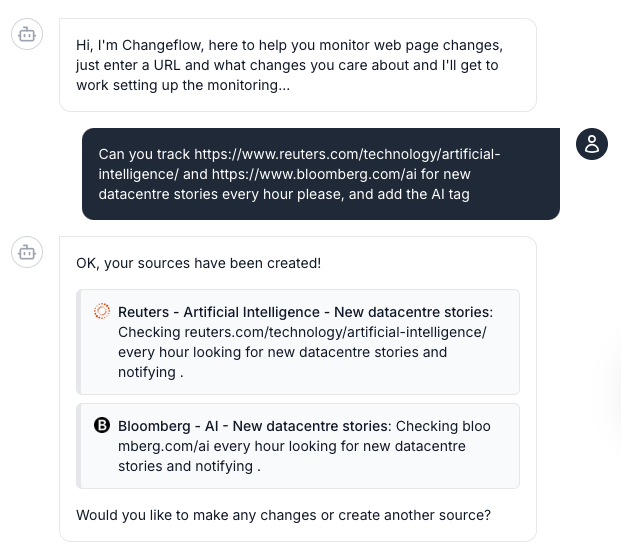
Stop manually checking FDA.gov every day. Set up monitoring in 60 seconds with natural language prompts like 'Track FDA guidance for oncology drugs' and let AI handle the rest.
- ✓ Monitor drug approvals, 510(k) clearances, and biologics licenses
- ✓ Track warning letters and enforcement actions by company or product
- ✓ Get AI summaries of guidance document changes
- ✓ Audit-ready timestamped archives of every regulatory change
- ✓ Filter by therapeutic area, product type, or compliance topic
Built for pharmaceutical compliance teams
FDA-trained AI
Our AI understands FDA terminology, document types, and regulatory language. It knows the difference between a draft guidance and a final rule.
Natural language setup
No technical configuration needed. Just tell us: 'Monitor FDA warning letters for pharmaceutical manufacturing' and we handle the rest.
Compliance summaries
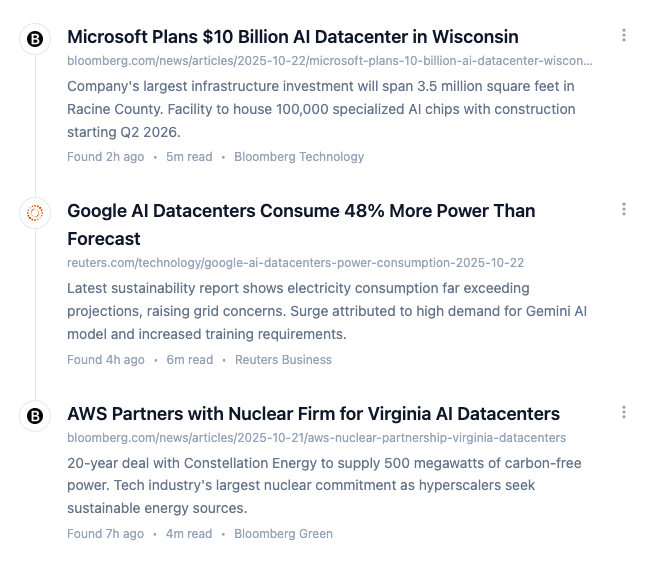
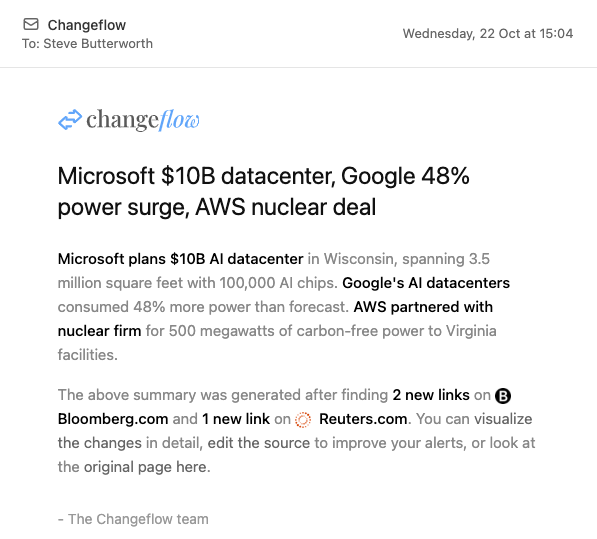
Get plain-language summaries of what changed and potential compliance implications. Save hours of reading dense regulatory documents.
Site Version Control
Timestamped, tamper-proof archives of every FDA page change. Perfect for audit trails and regulatory inspections.
Self-healing monitors
When FDA.gov redesigns (and they do), Changeflow automatically adapts. No broken monitors or missed changes.
Reliable monitoring
Advanced technology ensures consistent access to FDA.gov even when other tools get blocked or rate-limited.
Optimized for FDA and US regulatory sources
Changeflow has been specifically optimized to reliably monitor FDA.gov and related US healthcare regulatory sources. Our AI understands FDA document structures and terminology.
FDA.gov
fda.gov
FDA Drugs
fda.gov/drugs
FDA Devices
fda.gov/medical-devices
CMS
cms.gov
CDC
cdc.gov
NIH
nih.gov
HHS
hhs.gov
Federal Register
federalregister.gov
DEA
dea.gov
USP
usp.org
ICH
ich.org
State Boards
nabp.pharmacy
Plus any other FDA or healthcare regulatory source you need to track.
How pharma compliance teams use Changeflow
Guidance document tracking
Regulatory Affairs teams
Challenge: FDA publishes hundreds of guidance documents annually. Missing a relevant one can lead to compliance gaps or competitive disadvantage.
Solution: Set up AI-filtered monitoring for your therapeutic areas: 'Track FDA guidance for biosimilars' or 'Monitor CDER guidance for generic drugs.'
Outcome: Stay ahead of regulatory changes without dedicating staff to manual FDA.gov monitoring.
A mid-size pharma company now catches relevant guidance documents within hours of publication, giving them a 2-week head start on compliance planning.
Warning letter monitoring
Quality Assurance teams
Challenge: FDA warning letters to competitors reveal industry compliance trends and inspection priorities. Missing these insights puts you at risk.
Solution: Monitor FDA warning letters filtered by industry, violation type, or specific companies. Get alerts when competitors receive enforcement actions.
Outcome: Proactively address compliance gaps before FDA inspectors arrive at your facility.
A contract manufacturer identified a pattern of 483 observations in their sector and proactively updated procedures, avoiding similar citations.
Drug approval tracking
Commercial and competitive intelligence teams
Challenge: Tracking competitor drug approvals, NDA submissions, and pipeline developments requires constant FDA.gov monitoring.
Solution: Set up monitoring for CDER approvals, BLA submissions, and competitor company filings. AI filters to your therapeutic areas.
Outcome: Get competitive intelligence alerts within hours, not days or weeks.
An oncology-focused biotech now gets same-day alerts on all FDA oncology approvals, informing their commercial strategy.
Medical device clearances
Medical device regulatory teams
Challenge: Tracking 510(k) clearances, PMA approvals, and device recalls requires monitoring multiple FDA databases.
Solution: Monitor FDA device databases with AI filtering for your product categories, competitors, or predicate devices.
Outcome: Complete visibility into the device regulatory landscape affecting your products.
A cardiovascular device company tracks all 510(k) clearances in their category, identifying new competitors within days of FDA clearance.
Automated web intelligence
A URL and brief description of what you care about is all you need.
1 Describe what matters
Tell our AI agent what URLs to monitor and a brief description of what updates you want to be told about. No technical setup or manual configuration required.
2 Let our AI agent track the pages
The platform navigates to pages, checks for updates and uses AI to determine the relevance of the changes. Your personalized feed surfaces only what matters.
Simple pricing, for your team
Our software is 100% focussed on business. All plans offer comprehensive features and support.
Plus
Ideal for getting started with change detection, 20 to 300 pages monitored.
$14/mo
-
Track
20pages
- Email & chat support
- Onboarding calls & fast troubleshooting
- Hourly, daily & weekly checks
- 10 versions saved
- 2 notification addresses
- Centralized user admin & billing
Business
Ideal for teams that need to monitor changes on more web resources.
$14/mo
-
Track
20pages
- Everything in Plus, plus
- Expert email & chat support
- 20 versions saved
- 5 notification addresses
- Import from CSV, Excel & Site crawl
- Premium page unblocker
Enterprise
Ideal for teams that need to monitor and organise many web sources.
from$299/mo
-
Track
unlimitedpages
- Everything in Business, plus
- Allows 10 minute check frequency
- 50 versions saved
- 10 users included
- 40 notification addresses
- Concierge setup service
- Dedicated support, training & onboarding
- Custom plans & payment methods
Have a question? Get in touch. Not ready to pay? Try for free.
Frequently asked questions
If you can't find what you're looking for, email our support team and we'll get back to you with answers quickly.
-
Which FDA pages can Changeflow monitor?
Changeflow can monitor any page on FDA.gov including drug approvals, guidance documents, warning letters, 510(k) clearances, PMA approvals, recalls, and Federal Register notices. We also monitor related sites like CMS.gov, CDC.gov, and state health departments.
-
How quickly will I be notified of FDA changes?
Most clients set up hourly or daily monitoring for critical FDA pages. You'll typically receive alerts within 1-2 hours of FDA publishing changes, depending on your monitoring frequency settings.
-
Can I filter FDA updates by therapeutic area?
Yes! Use natural language prompts like 'Monitor FDA oncology guidance' or 'Track FDA approvals for diabetes drugs.' Our AI understands therapeutic areas and filters accordingly.
-
Is Changeflow compliant with FDA 21 CFR Part 11?
Changeflow's Site Version Control feature creates timestamped, tamper-proof archives suitable for regulatory audit trails. While we don't claim 21 CFR Part 11 certification, our archives provide the documentation many compliance teams need for inspections.
-
Can I monitor FDA warning letters for specific companies?
Absolutely. Set up monitoring for specific competitors' warning letters, or filter by violation type, facility type, or geographic region. Many clients use this for competitive intelligence and proactive compliance.
-
How does pricing work for FDA monitoring?
Changeflow pricing is based on the number of pages you monitor. Most pharma compliance teams start with our Business tier ($119-$269/month) for 400-900 pages. Enterprise plans with Site Version Control start at $299/month.
-
What if FDA.gov redesigns or changes structure?
Our self-healing monitoring technology automatically detects and adapts to site changes. You won't experience broken monitors or missed updates when FDA updates their website.
-
Can I get alerts via email, Slack, or other channels?
Yes. Changeflow supports email alerts, Slack notifications, Microsoft Teams, webhooks, and RSS feeds. Most compliance teams integrate with their existing workflow tools.
START MONITORING FDA CHANGES TODAY
Join pharmaceutical teams who never miss an FDA update
- 30-day free trial, no credit card required
- Setup takes 60 seconds with AI assistance
- Trusted by Pfizer and leading pharma companies
Questions? Our specialists are here to help, just email [email protected]